2-YEAR DATA FROM APEX (mITT POPULATION): A PROVEN EFFECTIVE TREATMENT PATHWAY
Demonstrated efficacy with or without cataract surgery10
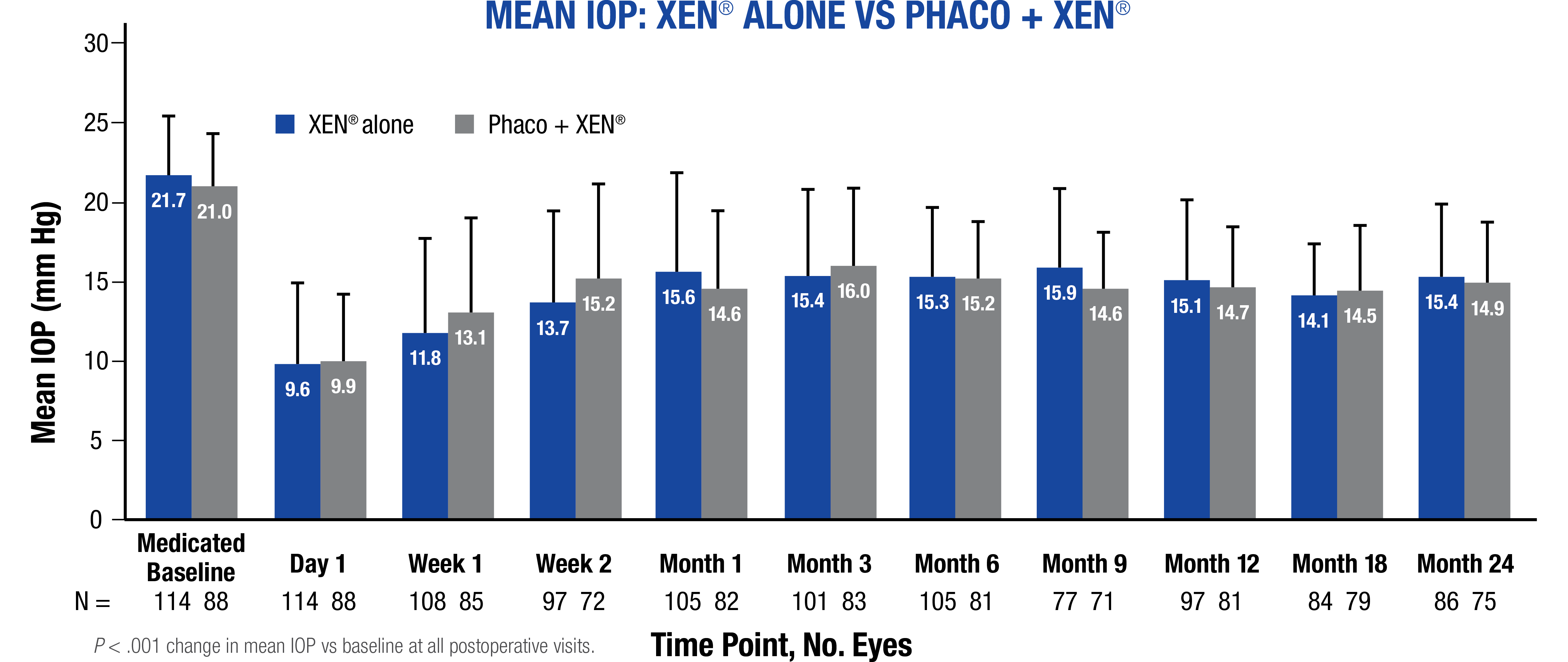
- Changes in mean IOP and mean topical IOP-lowering medications were statistically significant at all postoperative visits (P < .001)10
Prospective, nonrandomized, open-label, multicenter, 2-year European study evaluated the effectiveness of XEN® Gel Stent as primary surgical intervention in reducing IOP and IOP-lowering medication count in 200 patients (219 eyes) with medically uncontrolled, moderate primary open-angle glaucoma. Eyes with medicated baseline IOP 18 to 33 mm Hg on 1 to 4 topical IOP-lowering medications were implanted with XEN® (n = 120) or phaco + XEN® (n = 98). The mITT population included all enrolled eyes that received a XEN® Gel Stent and met the IOP and IOP-lowering medication count inclusion criteria.10
mITT = modified intent-to-treat; phaco = phacoemulsification with intraocular lens replacement.
APEX results: mean IOP in the low-to-mid teens through year 210
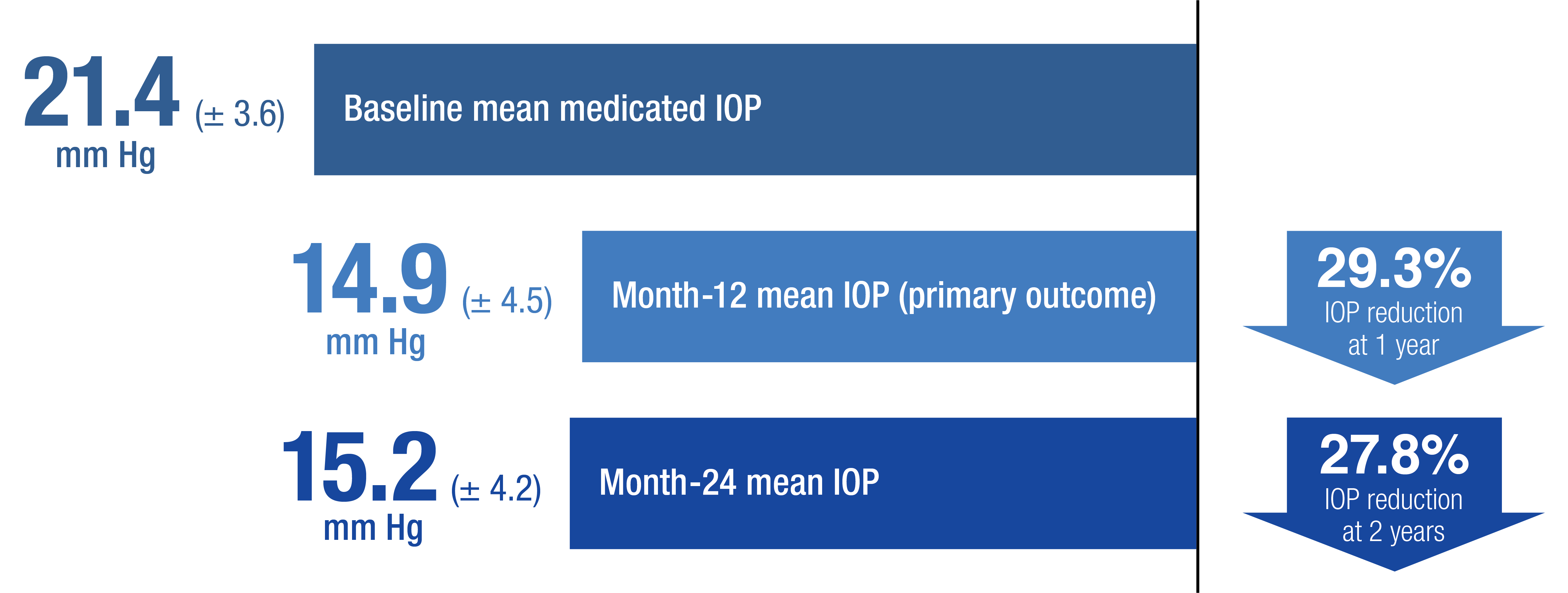
- The change in mean IOP from medicated baseline was -6.5 ± 5.3 at month 12 and -6.2 ± 4.9 at month 24 (P < .001)10
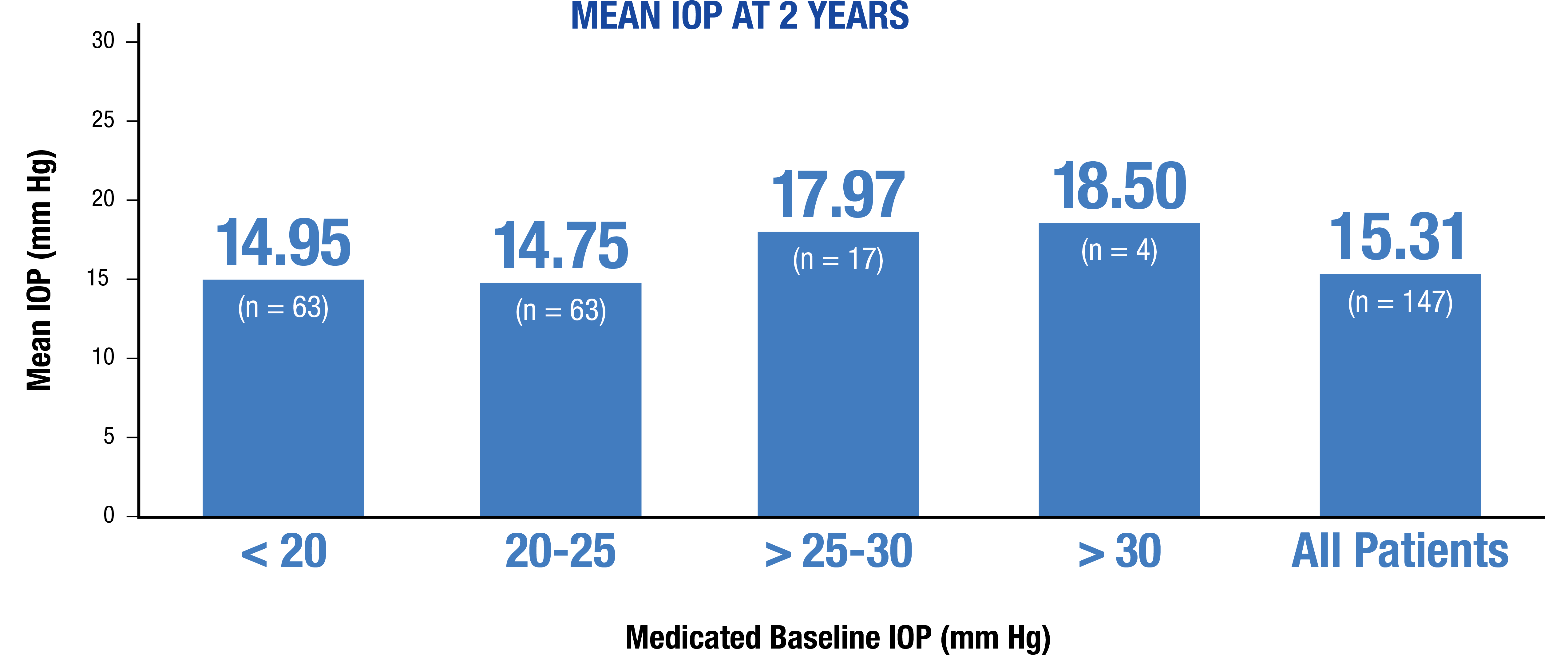
Demonstrated efficacy from a range of baseline pressures17
≥ 20% reduction in IOP on same or fewer topical medications10
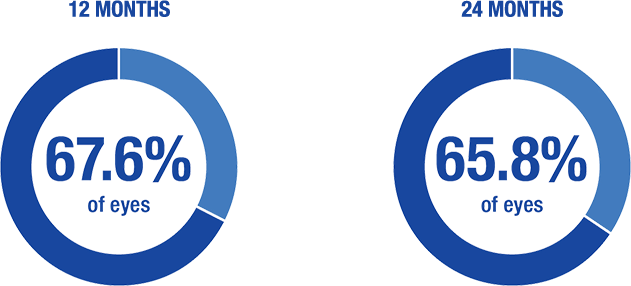
- 83.7% and 85.1% of eyes ≤ 18 mm Hg IOP at 12 months and 24 months, respectively
- 60.7% and 62.7% of eyes ≤ 15 mm Hg IOP at 12 months and 24 months, respectively
XEN® Gel Stent reduced or eliminated the need for topical IOP-lowering medications vs baseline10,*

*A reduction of 1.7 (± 1.3) in the mean number of IOP-lowering medications vs baseline was reported at month 12 (primary outcome; P < .001) and a reduction of 1.5 (± 1.4) was reported at month 24 (P < .001).
Many patients were medication free for the entire 2-year study10

APEX study safety data10
Intraoperative Complications (10/218)
4.6% Anterior chamber bleeding in 2.8% (6/218) of eyes was the most common
Postoperative ocular adverse events (65/218)
29.8% of eyes had 1 or more Glaucoma-related SSI due to uncontrolled IOP (14/218; 6.4%) and hyphema (10/218; 4.6%) were most frequently reported
Persistent hypotony (0/218)
0% Defined as IOP < 6 mm Hg at 2 consecutive postoperative visits > 30 days apart
Numeric Hypotony (44/218)
20.2% Defined as IOP < 6 mm Hg (self-resolved) within the first 2 postoperative weeks
2.3% (5/218) were recorded as adverse events, all of which occurred within 1 week post implantation and self-resolved within 1 month
Ocular Serious Adverse Events (6/218) (5 in study eyes, 1 in untreated fellow eye)
2.8% Cataract aggravated, retinal disorder (central retinal vein occlusion reported at 12 months, without elevated IOP), conjunctival erosion (implant exposure), glaucoma-related SSI (due to hospitalization for surgery), endophthalmitis (reported 15 months after implantation), and high IOP with SSI (cyclodestructive procedure) in the untreated fellow eye (n = 1 each).
APEX study limitations10
- Variability in perioperative regimens may have impacted study outcomes. At the time of initiation of this study, XEN® Gel Stent was new on the market and no best practices were established, so the study results also reflect the investigators’ learning curve with the surgery and the variation in preoperative and postoperative regimens associated with typical clinical settings
- The study population underrepresented patients who were not White; < 5% were Asian or Black/African ethnicities. Black/African and Asian ethnicities have been reported to have increased risk for trabeculectomy failure
- The APEX study design was nonrandomized and open label, which does not permit direct comparison to other MIGS and surgical techniques
This study was funded by AbbVie. Herbert Reitsamer has received consulting honoraria from AbbVie. Chelvin Sng has received consulting honoraria from AbbVie, Glaukos (San Clemente, California), Alcon (Fort Worth, Texas), and Santen Pharmaceuticals (Osaka, Japan). Vanessa Vera has received consulting honoraria from AbbVie. Keith Barton has received consulting honoraria from Alcon, AbbVie, and Glaukos. Ingeborg Stalmans has received consulting honoraria from AbbVie. Markus Lenzhofer declares that he has no conflict of interest.
