6-mm length, 45-micron lumen diameter—about the length of an eyelash1,8
A STANDARDIZED PROCEDURE TO CREATE A NEW AQUEOUS OUTFLOW PATHWAY

The first durable, biocompatible gel stent1,3
- Made of durable, biocompatible, tissue-conforming gelatin that becomes flexible after implantation4,5
- Biocompatible gelatin has an extensive history in medical devices and tissue engineering, with over 45 years of proven clinical use5
- XEN® Gel Stent has been the trusted choice of surgeons more than 120,000 times in its 7 years post clearance in the US6

Preloaded, disposable injector with a 27-gauge, double-beveled needle1,7
Bypasses diseased trabecular tissue1
- Implanted through a corneal microincision using an ab interno approach1
- Creates a pathway for aqueous outflow to the subconjunctival space1
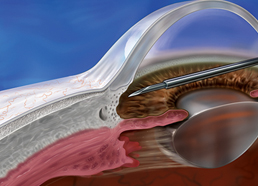
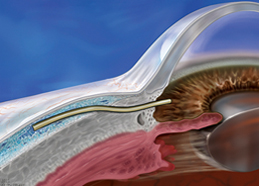
In the XEN® Gel Stent clinical study, standard ophthalmic surgery techniques, viscoelastic, and mitomycin C (0.2 mg/mL by sponge application) were used before injection.1
