INDICATIONS
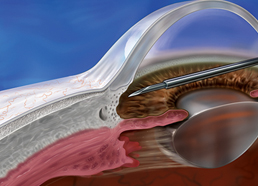
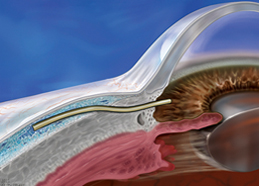
The XEN® Glaucoma Treatment System (XEN® 45 Gel Stent preloaded into a XEN® Injector) is indicated for the management of refractory glaucomas, including cases where previous surgical treatment has failed, cases of primary open-angle glaucoma, and pseudoexfoliative or pigmentary glaucoma with open angles that are unresponsive to maximum tolerated medical therapy.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
XEN® Gel Stent is contraindicated in angle-closure glaucoma where angle has not been surgically opened, previous glaucoma shunt/valve or conjunctival scarring/pathologies in the target quadrant, active inflammation, active iris neovascularization, anterior chamber intraocular lens, intraocular silicone oil, and vitreous in the anterior chamber.
WARNINGS
XEN® Gel Stent complications may include choroidal effusion, hyphema, hypotony, implant migration, implant exposure, wound leak, need for secondary surgical intervention, and intraocular surgery complications. Safety and effectiveness in neovascular, congenital, and infantile glaucoma has not been established. Avoid digital pressure following implantation of the XEN® Gel Stent to avoid the potential for implant damage.
PRECAUTIONS
Examine the XEN® Gel Stent and XEN® Injector in the operating room prior to use. Monitor intraocular pressure (IOP) postoperatively and if not adequately maintained, manage appropriately. Stop the procedure immediately if increased resistance is observed during implantation and use a new XEN® system. Safety and effectiveness of more than a single implanted XEN® Gel Stent has not been studied.
ADVERSE EVENTS
The most common postoperative adverse events included best-corrected visual acuity loss of ≥ 2 lines (≤ 30 days 15.4%; > 30 days 10.8%; 12 months 6.2%), hypotony IOP < 6 mm Hg at any time (24.6%; no clinically significant consequences were associated, no cases of persistent hypotony, and no surgical intervention was required), IOP increase ≥ 10 mm Hg from baseline (21.5%), and needling procedure (32.3%).
Caution: Federal law restricts this device to sale by or on the order of a licensed physician. Please call 1-800-433-8871 to report an adverse event.
Please see accompanying full Directions for Use or visit https://www.rxabbvie.com/pdf/xen_dfu.pdf
US-XEN-230091