The first ab interno approach to create a new pathway for aqueous outflow1
THINK XEN® AT THE POINT OF YOUR SURGICAL DECISION.
Demonstrated to reduce IOP for a broad range of refractory glaucoma patients1
Established efficacy and safety data1
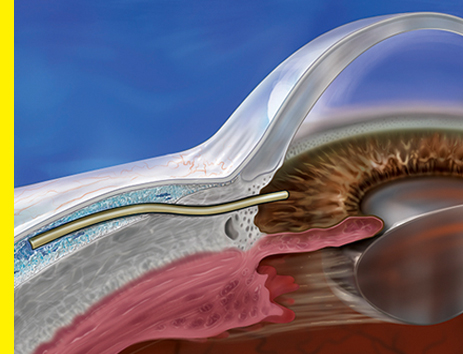
DESIGNED FOR EFFICACY
XEN® Gel Stent is the only FDA-cleared stent that creates a low-lying, ab interno bleb for effective intraocular pressure (IOP) reduction.2
PATIENT SELECTION
XEN® Gel Stent is indicated for refractory glaucoma patients, including those who are1:
- Pseudophakic
- Uncontrolled on maximum tolerated medical therapy
- Inadequately controlled by prior surgery, including MIGS and SLT*
- Undergoing cataract surgery or a stand-alone procedure
*In the clinical study, prior surgery only included trabeculectomy, tube shunt, canaloplasty, selective laser trabeculoplasty (SLT), and trabeculotomy.
Pseudophakic (with or without prior MIGS)*
Inadequately controlled by prior surgery including MIGS*
Uncontrolled on maximum tolerated medical therapy
Undergoing cataract surgery (can be performed in combination with XEN® Gel Stent)
MIGS = minimally invasive glaucoma surgery.
DOCUMENTED XEN® GEL STENT OUTCOMES
In the primary analysis at 12 months, 76.3% (95% CI = 65.8, 86.8%) of subjects achieved ≥ 20% mean diurnal IOP reduction on the same or fewer number of medications vs baseline (N = 65).1
PROVEN SAFETY DATA
Learn more about the proven safety data of XEN® Gel Stent through pivotal safety data and the 2-year APEX study.
